Development and approval
Two COVID-19 vaccines have already been approved, and others could follow in the course of the year. What stage are the projects at? What quantities of vaccines will Germany receive?
2 min reading time
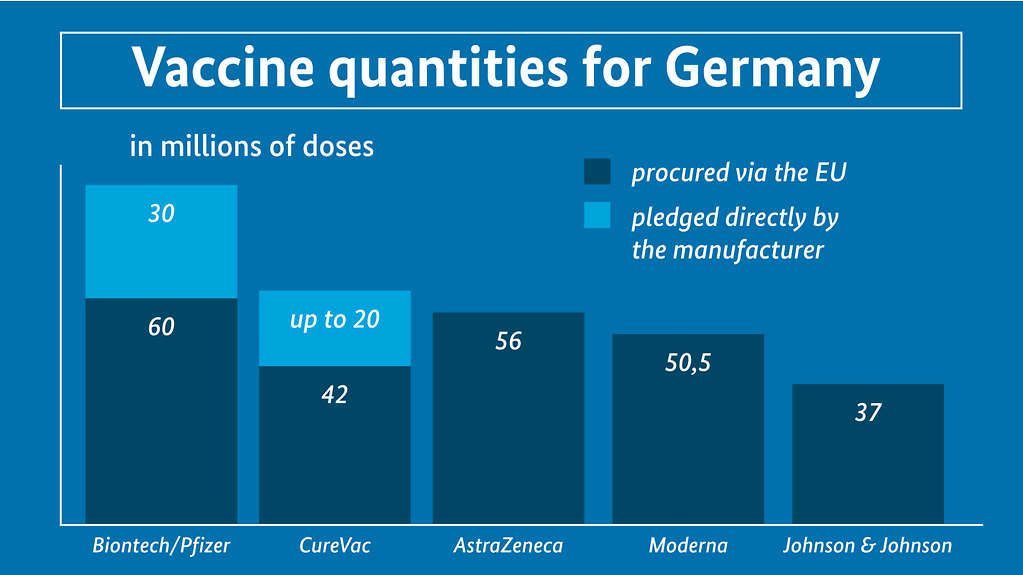
The agreed quantities of approved and potential vaccines for Germany
The diagram is entitled ‘Vaccine quantities for Germany in millions of doses. Beside this we see a key: first colour block – procured via the EU; second colour block – pledged directly by the manufacturer. Below this are five bars: BioNTech/Pfizer: quantity pledged via EU 60; quantity pledged by manufacturer: 30; CureVac: quantity pledged via EU: 42; quantity pledged by manufacturer: up to 20; AstraZeneca: quantity pledged via the EU: 56; Moderna: quantity pledged via the EU: 50.5; Johnson&Johnson: quantity pledged via the EU: 37
Photo: Bundesregierung
The BioNTech/Pfizer vaccine was approved for use in the EU on 21 December 2020, followed on 6 January 2021 by the Moderna vaccine. Other projects are already at an advanced stage. Germany has secured large quantities of both of the vaccines already approved and of promising vaccine candidates. Here is an overview of the current stages of development of the various vaccines:
- BioNTech/Pfizer: a minimum of 60 million doses via the EU plus a guaranteed option to purchase another 30 million doses at national level
(current status: vaccine approved for use) - Moderna: 50.5 million doses via the EU; negotiations for addition doses ongoing at national level
(current status: vaccine approved for use) - AstraZeneca: 56 million doses via the EU
(current status: all data packages relevant for approval submitted to the European Medicines Agency (EMA), application for approval lodged on 12 January 2021) - Johnson&Johnson (developed by subsidiary Janssen): 37 million doses via the EU
(current status: initial data packages relating to the phase three study currently being considered by the EMA, no application for approval yet lodged) - CureVac: minimum of 42 million doses via the EU plus the option to purchase 20 million doses at national level
(current status: phase three study launched in December; no EMA review to date)
On behalf of the member states, the European Commission entered into contracts with manufacturers. During a global pandemic, unilateral actions at national level hamper effective health protection. "We very deliberately chose to act jointly with our partners in the European Union," stressed federal government spokesperson Steffen Seibert.
The European Medicines Agency (EMA) offers a comprehensive overview of the current state of development of a whole series of COVID-19 vaccines. According to the World Health Organization (WHO), 63 vaccine candidates are currently being tested on volunteers within the scope of clinical studies around the world. The WHO also provides an overview.
Our detailed FAQs provide answers to important questions relating to vaccinations.